Abstract
Age related clonal haematopoiesis (ARCH) is defined as the clonal expansion of hematopoietic stem cells (HSCs), driven by recurrent mutations. Many of these are also commonly seen in haematological malignancies, hence termed pre-leukemic mutations (pLM). ARCH carries an increased risk of haematological malignancies and cardiometabolic disease. Further understanding of the role of pLMs in HSC function is necessary to predict and possibly prevent leukemia. DNMT3A R882 is the most common pLM observed in ARCH and is associated with poor outcomes in acute myeloid leukemia (AML). It is acquired early in life and is positively selected in HSCs. HSCs of Dnmt3a knock-out or knock-in mouse models have increased self-renewal capacity and can differentiate towards all lineages. DNMT3A R882 mutation is observed in all mature blood lineages in ARCH individuals, indicating their multipotential differentiation capacity, but the dynamics of differentiation are not known. We hypothesise that DNMT3A R882 mutation affects the differentiation dynamics of HSCs, potentially contributing to disease risk.
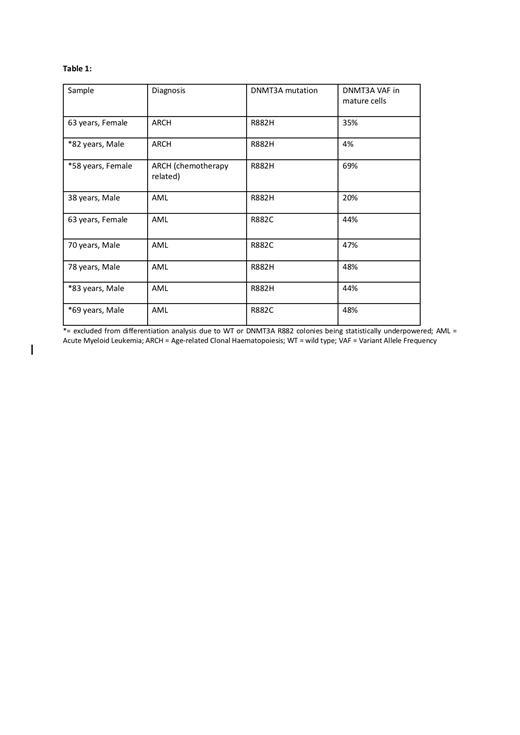
We characterised the functional changes in HSC differentiation driven by DNMT3A R882 at single cell resolution. Single phenotypic HSCs (CD33-/CD34+/CD45dim/CD38-/CD45RA-) from 9 individuals (Table 1) were cultured in media supporting differentiation into all major blood lineages. To assess changes in differentiation capacity between DNMT3A R882 and wild-type (WT) HSCs within each individual, each single-cell colony was genotyped by targeted DNA sequencing and scored by high-throughput flow cytometry. Flow cytometry data was analysed using a novel unbiased analytical pipeline, which we have named 'FlowPAC', allowing generation of a two-dimensional representation of the global output of HSC differentiation by identifying clusters based on fluorochrome intensity. Four samples were underpowered for either DNMT3A R882 or WT colonies and were excluded from this analysis.
We did not observe any difference in erythroid versus myeloid or lymphoid (NK) differentiation capacity between DNMT3A R882 and WT HSC. DNMT3A R882 and WT HSCs also produced similar proportions of monocytic and neutrophil colonies. However, FlowPAC analysis identified differences in their level of maturity. CD15 (neutrophil marker) expression was significantly increased in myeloid clusters of DNMT3A R882 colonies compared to WT (p=0.0043), whereas CD14 (monocyte marker) expression was decreased compared to WT (p=0.0645). We further analysed mature neutrophil differentiation using CD66b expression. Consistent with promotion of neutrophil differentiation, CD66b median fluorescence intensity was significantly increased in the CD15+ population of DNMT3A R882 colonies compared to WT. To further investigate monocyte differentiation, we performed RNAseq on colonies containing only monocytes from 1 ARCH sample. Analysis of transcriptional profiles confirmed less mature monocytes in DNMT3A R882 colonies compared to WT. Overall, our data indicate that DNMT3A R882 mutation in HSCs alters neutrophil and monocyte production.
Additionally, we observed that in contrast to samples with high LSC content leading to leukemic engraftment in mice, in samples where DNMT3A R882 HSCs were able to reconstitute normal blood production, the expression of the cell surface marker CD49f, a marker of the most potent HSCs, was significantly increased in DNMT3A R882 HSCs compared to WT at the time of sorting. This may suggest unequal distribution of this pLM within the preserved normal HSC pool prior to leukemic transformation.
In conclusion, DNMT3A R882 alters the dynamics of human HSC myeloid differentiation. The propensity of DNMT3A R882 derived monocytes to retain an immature phenotype relative to WT cells could represent an early change later facilitating the complete differentiation block seen in AML, driven by the acquisition of additional mutations such as NPM1. DNMT3A R882 also promoted neutrophil maturation. Altered neutrophil differentiation has been observed in patients with germline DNMT3A mutation. As altered neutrophil function plays a key role in cardiovascular disease, future studies are required to investigate whether the effect of DNMT3A R882 on neutrophil differentiation may contribute to the increased cardiovascular disease risk associated with this mutation.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal